All present
Competition in the pouch segment has heated up significantly, and manufacturers are increasing production capacity for their “modern oral” products.
It all started with the introduction of Zyn by Swedish Match, the world’s largest producer of snus in Sweden and a significant player in the U.S. market for smokeless products. In the first quarter of 2020, Zyn was sold in 13 countries outside of the U.S. and Scandinavia, including many EU member states, such as Austria, Croatia, Denmark, Germany, the U.K. and the Czech Republic.
Growth of Zyn has been so strong that Swedish Match CEO Lars Dahlgren spoke of a “transformational year” for the company, saying that the product had been the key driver of the company’s U.S. smoke-free sales in 2019. In the first quarter of 2020, Swedish Match sold almost 70 million cans of Zyn in the U.S., up from roughly 18 million cans in the same period a year earlier. Swedish Match also filed a premarket tobacco product application (PMTA) with the U.S. Food and Drug Administration (FDA).
Demand for Zyn has been so strong that Swedish Match announced expansions of its U.S. production facility twice in short succession. Scheduled for completion in 2020, the fourth phase of expansion will increase capacity to more than 200 million cans per year.
Swedish Match’s competitors have not been idle. By acquiring an 80 percent stake in the global business of Burger Soehne, Altria in June 2019 became the owner of the On! nicotine pouch brand. Altria will provide global distribution for On!, which currently is available at retail outlets in the U.S., Canada, Sweden and Japan as well as globally through the company’s online shop. On! comes in seven flavors, including coffee, berry and citrus, and five different nicotine strengths. In May 2020, Altria submitted a PMTA for 35 On! products to the FDA.
British American Tobacco (BAT) is represented in the nicotine pouch market through its Lyft brand, which it sells in the U.K., Sweden and Kenya. Going forward, BAT plans to market all its modern oral products under the name Velo—the brand under which BAT subsidiary Reynolds American Inc. (RAI) has been marketing its nicotine pouch product in the U.S. since June 2019.
To cater to the anticipated increase in demand, BAT in September 2020 built a nicotine pouch factory in Hungary. The investment, estimated at more than HUF7.5 billion ($24.3 million), the investment aims to boost production to more than 1 billion nicotine pouches in 2020, a figure that is expected to triple next year. Initially equipped with one line for the manufacture of nicotine pouches, the factory is supposed to receive a further five production lines by the end of this year. Its output is destined mainly for European markets, including Germany, Austria and the Nordic countries. The Hungarian factory is supposed to become one of BAT’s global hubs for the manufacturing of oral products.
In June last year, Japan Tobacco International (JTI) entered the race with Nordic Spirit, nicotine pouches that were developed in Sweden and sold in Switzerland and Sweden and online. Upon the launch, the company had said it intends to significantly increase distribution across various trade channels in the near term. Nordic Spirit is currently available in four flavors, including mint and bergamot wild berry.
Imperial Brands is present in the modern oral nicotine market with Zone X, Killa, BLCK and Pablo, among other products. With a high nicotine content of 50 mg per gram, Pablo is the “strongest” nicotine pouch in the market. Usually, nicotine content in the novel products vary between 2 mg and 24 mg per gram.









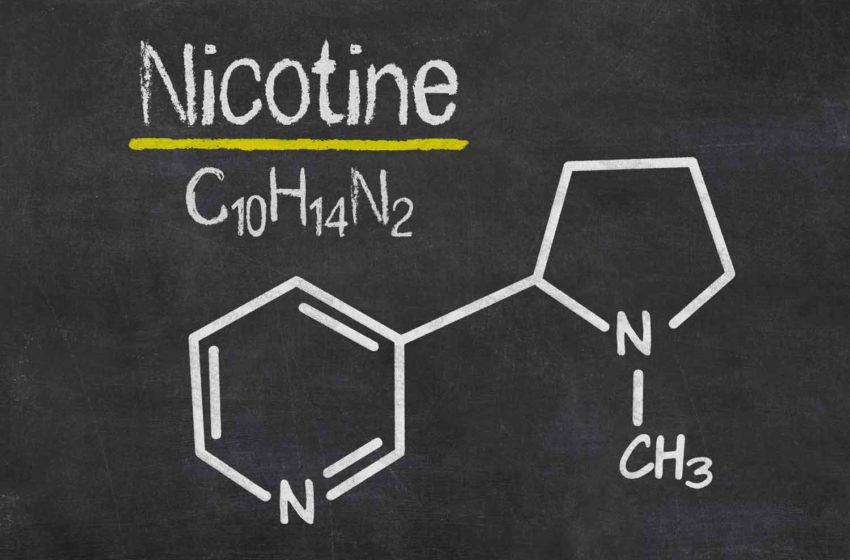
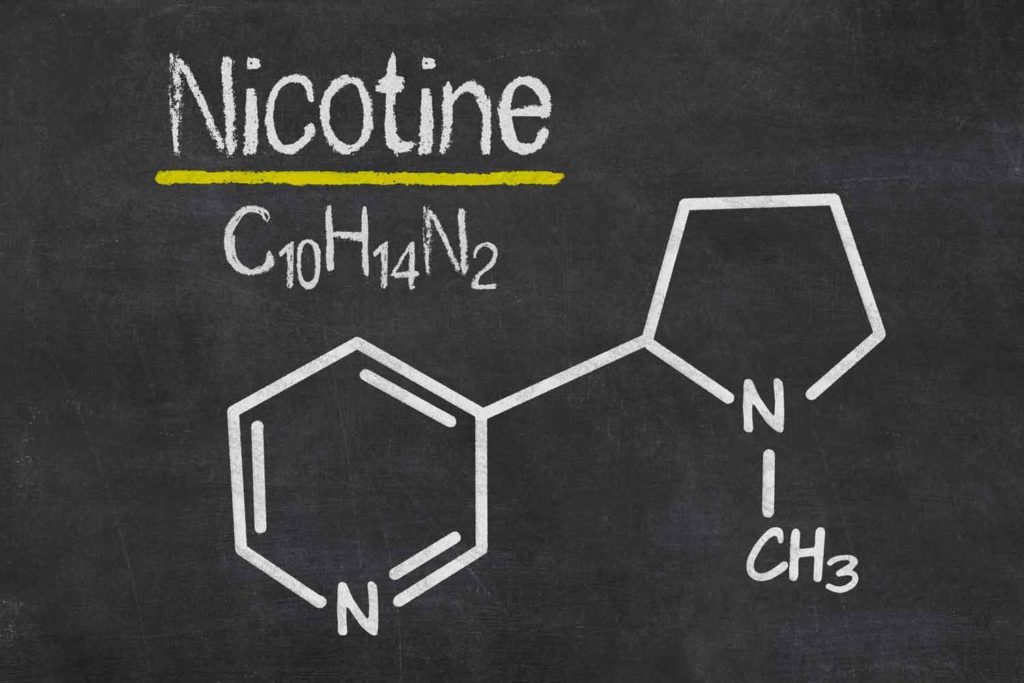



 Kaival Brands Innovations Group is restarting production of its Bidi Pouch ahead of an anticipated September launch.
Kaival Brands Innovations Group is restarting production of its Bidi Pouch ahead of an anticipated September launch.
 The study, published in
The study, published in 



