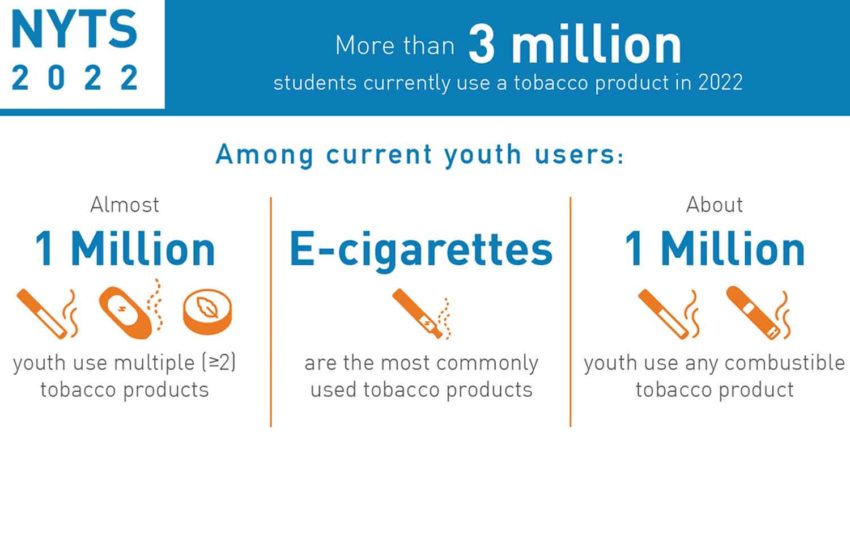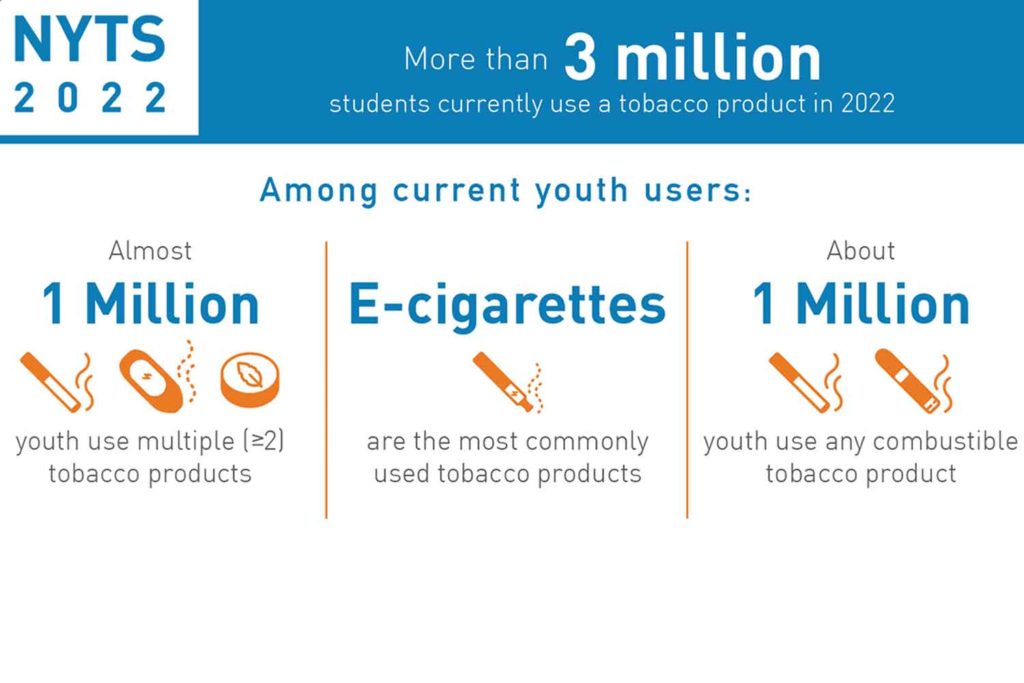
More than one in 10 U.S. middle school and high school students (3.08 million) had used a tobacco product during the past 30 days in 2022, according to data from the 2022 National Youth Tobacco Survey (NYTS) released by the U.S. Food and Drug Administration and Centers for Disease Control and Prevention in the Morbidity and Mortality Weekly Report. This figure included 16.5 percent of high school students and 4.5 percent of middle school students.
Approximately 3.7 percent (1 million) of all students reported currently smoking any combustible tobacco product. Current use of at least two tobacco products was reported by 3.5 percent (960,000).
The most commonly used type of product were e-cigarettes (9.4 percent), followed by cigars (1.9 percent) and cigarettes (1.6 percent). More than 2.5 million middle school and high school students currently used e-cigarettes.
“It’s clear we’ve made commendable progress in reducing cigarette smoking among our nation’s youth. However, with an ever-changing tobacco product landscape, there’s still more work to be done,” said Brian King, director of the FDA’s Center for Tobacco Products. “We must continue to tackle all forms of tobacco product use among youth, including meaningfully addressing the notable disparities that continue to persist.”
Additionally, the 2022 NYTS findings suggest ongoing disparities in tobacco product use—both overall and across population groups. For example, current use of any tobacco product was higher among non-Hispanic American Indian or Alaska Native students (13.5 percent), those identifying as lesbian, gay or bisexual (16 percent), transgender (16.6 percent), and those experiencing severe psychological distress (12.5 percent). Non-Hispanic Black students reported the highest percentage of combustible tobacco product use (5.7 percent), including cigar use (3.3 percent).
The NYTS is a nationally representative survey of U.S. middle school (grades 6–8) and high school (grades 9–12) students that focuses exclusively on tobacco product use and associated factors and remains critical to informing the FDA’s tobacco regulatory activities in the United States. The FDA and the CDC have been collaborating on the implementation of the NYTS questionnaire and release of data since 2011.