R.J. Reynolds Vapor Co. owes Philip Morris Products more than $14 million after a federal judge on Aug. 17 increased a jury’s June patent-infringement award over vapor products to include prejudgment interest and supplemental damages, reports Bloomberg Law.
Judge Leonie M. Brinkema amended the judgment entered June 15 in the U.S. District Court for the Eastern District of Virginia to reflect a total judgment amount of $10.9 million for infringement of one patent and $3.16 million for infringement of another.
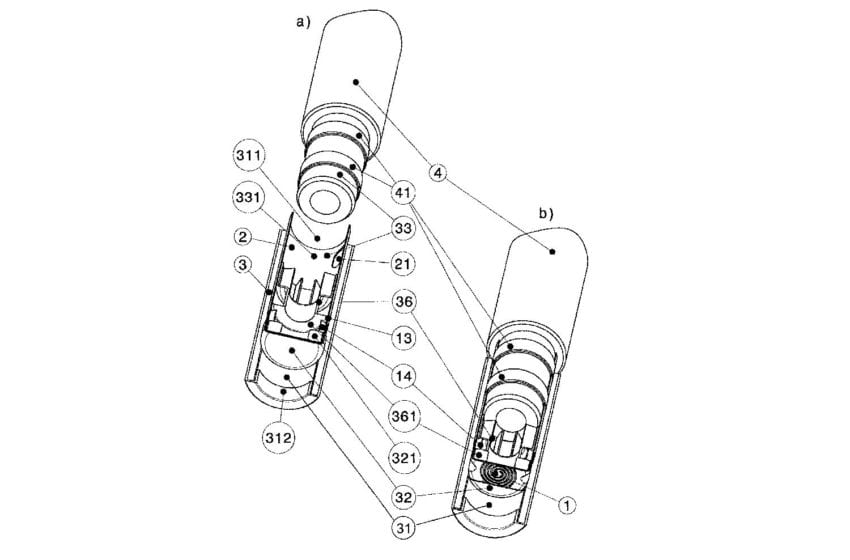
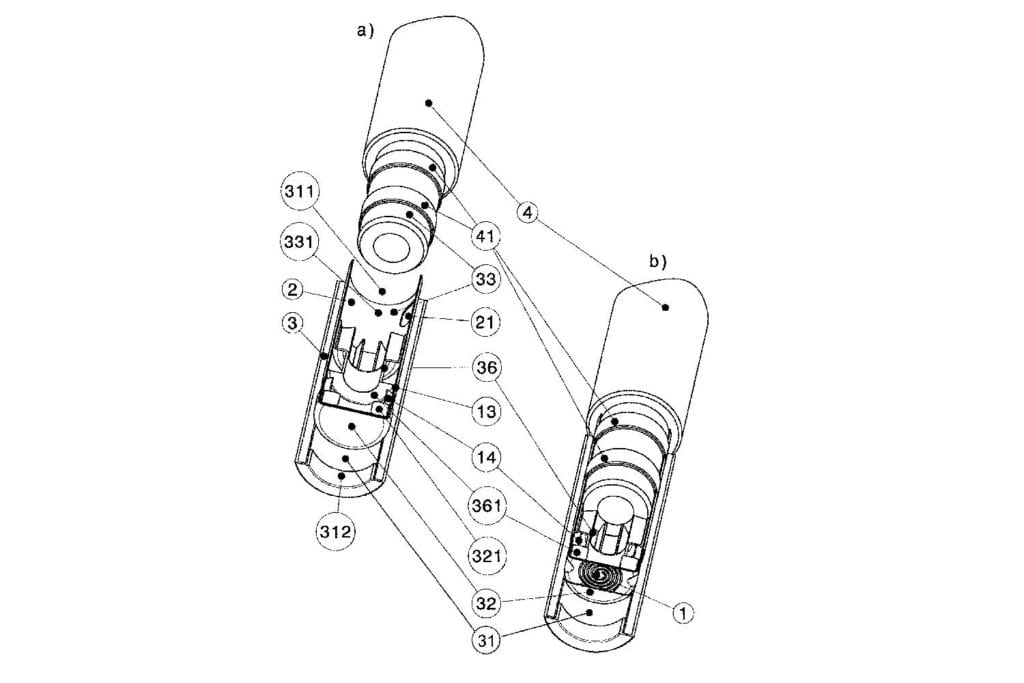
In its June 15 judgement, the jury found that RJR’s Vuse Solo and Alto devices infringe two Philip Morris patents covering parts of a vaping device for heating substances and preventing leaks. At the same time, the jury cleared Vuse Alto of infringing one of the patents.
The verdict concerned counterclaims in RJR’s ongoing patent lawsuit over PMI’s IQOS heated-tobacco device. RJR won an order blocking IQOS imports at the U.S. International Trade Commission last November.
Philip Morris succeeded earlier this year in invalidating parts of some patents RJR accused it of infringing at a U.S. Patent Office tribunal.
RJR parent company BAT has also sued Philip Morris over IQOS in the United Kingdom, Germany and elsewhere. A PMI filing with the U.S. Securities and Exchange Commission earlier this year said IQOS patent lawsuits and challenges outside of the U.S. have “repeatedly and universally failed.”
Altria has separately sued Reynolds for patent infringement in North Carolina over the Vuse line.