The Options
Over the past few months, many makers of flavored e-cigarette products have received marketing denial orders (MDOs) or fear receiving one. An MDO gives that manufacturer a limited number of choices: reapply for approval, get out of the business or sue the FDA for redress—or search for what it hopes is a legal loophole to the FDA’s regulatory authority. Hence, the move to synthetic nicotine as a too clever by half solution that carries with it underlying scientific, economic, legal and ethical issues.
Let’s start with an economic issue, which may trump all the others. Synthetic nicotine is expensive. In fact, it’s up to 10 times the cost of tobacco-derived nicotine, according to Kevin Burd, CEO of North America Nicotine. One reason is that tobacco-derived nicotine is made from leftover scraps from processing tobacco leaves—often literally the stuff left on the factory floor. Synthetic nicotine, most of which is manufactured in China, is created through a variety of proprietary chemical processes.
“As volumes increase, I can’t see the price of chemicals used in synthetic coming down to the price of waste tobacco, including dust,” said Burd. “It’s highly unlikely ever to be competitive.”
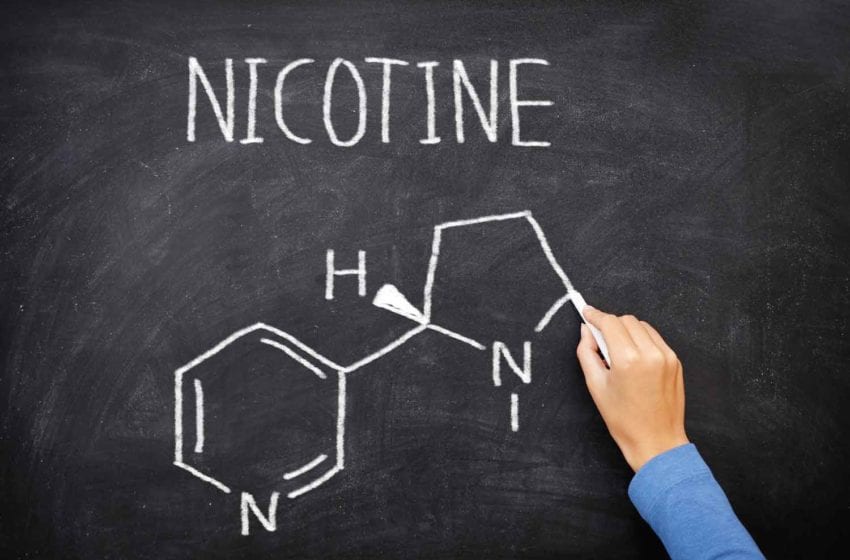
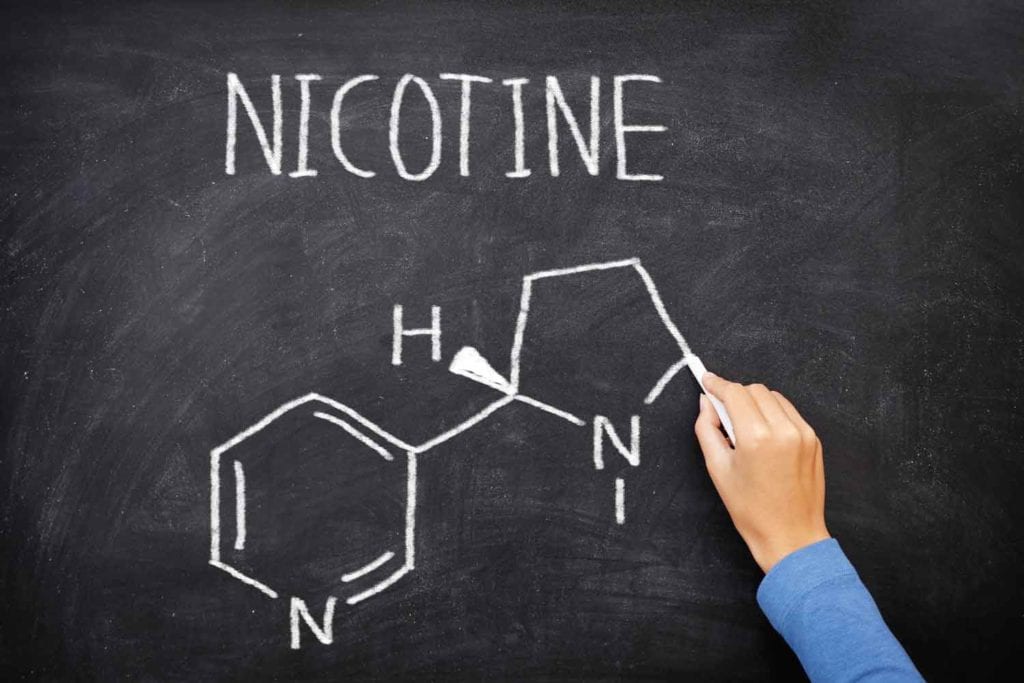
Both synthetic nicotine and tobacco-derived nicotine occur in two structural forms (enantiomers) known as R-nicotine and S-nicotine. More than 99 percent of the nicotine in tobacco is S-nicotine. In synthetic nicotine, that balance can be 50-50.
“Little is known about the pharmacological and metabolic effects of R-nicotine in humans,” says a 2021 article on the rise of synthetic nicotine in Tobacco Control (Jordt SE. Synthetic nicotine has arrived. Tobacco Control 2021. doi: 10.1136/tobaccocontrol-2021-056626). In other words, synthetic product users could be unwitting research subjects. There are inexpensive tests for distinguishing between R- and S- forms of the chemical and ways of separating the R-isomer from the S-isomer. By comparison, telling the difference between synthetic S-nicotine and tobacco-derived S-nicotine can be quite costly.
This leads to an interesting paradox. We don’t know how much of the supposed synthetic nicotine is actually tobacco-derived nicotine. There’s a financial incentive for a manufacturer simply to replace the more expensive version with the cheaper chemical and mislabel it. In fact, on its website, Next Generation Labs warns prospective customers, “There are several companies from China claiming to have synthetic nicotine. NGL has tested several of these products and discovered they are made or derived from tobacco.”
Burd adds, “Quite likely, many [products that claim to be synthetic] are not really synthetic.”
Some companies are weighing the cost of regulatory compliance versus the higher manufacturing cost of using synthetic nicotine. That’s a false choice, for it underestimates the costs of unknown safety risks, product testing and emerging regulations. Simply marketing a new synthetic nicotine product without providing the necessary scientific and behavioral research data to the FDA will, at the very least, generate a warning letter.
Remember that the key reason for generating research data on a new product is to explore whether it will harm people. The FDA uses the term APPH (appropriate for the protection of public health). Not doing so puts your business at risk not only from regulatory actions but from civil litigation if someone gets hurt.
A regulatory update by FDA Center for Tobacco Products Director Mitch Zeller at an Oct. 27 Food and Drug Law Institute conference addressed the challenge posed by synthetic nicotine. Zeller’s slide deck makes clear his belief that e-liquids that do not contain tobacco-derived substances “may still be components or parts of tobacco products and, therefore, subject to FDA’s tobacco control authorities.” It also states that the FDA is “in discussions with Congress about a potential legislative fix.”