Public Health England (PHE) said yesterday that going ‘cold turkey’ was the least effective way to quit tobacco smoking.
In a note to mark No Smoking Day, PHE said that over 58 percent of smokers still tried to quit without using an aid, despite this being the least effective way.
‘A Public Health England (PHE) report highlights that public misunderstanding of the harmfulness of nicotine-containing products, such as nicotine replacement therapy (NRT) and e-cigarettes, may be linked to inaccurate and confused perception of the risks of nicotine,’ the note said.
‘The risks of nicotine use are likely to be very low or negligible. NRT is safe and licenced for use in pregnancy and for people with cardiovascular disease. And there is now wide international consensus that e-cigarettes are far less harmful than smoking. It is the cocktail of deadly chemicals in cigarette smoke, including tar and carbon monoxide, which causes almost all of the harm of smoking.
‘Four in 10 smokers and ex-smokers incorrectly think that nicotine in cigarettes is the cause of most of the smoking-related cancer. Understanding of the harms of nicotine among the general population is similarly poor…’
The PHE note is at: https://www.gov.uk/government/news/four-in-10-smokers-incorrectly-think-nicotine-causes-cancer.
Tag: United Kingdom

Nicotine misunderstood

Reducing risk
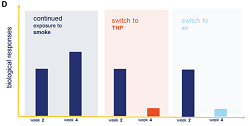
A new laboratory-based study has shown that when airway cells damaged by cigarette smoke are exposed instead to vapor from the heated-tobacco product, glo, some of the biological effects caused by the smoke exposure are reversed.
Basically, subsequent exposure of cigarette-smoke-impacted cells to glo vapor had a similar effect to their subsequent exposure to air – the cells were able to maintain their ability to repair themselves.
In a press note, British American Tobacco said that the vapor produced by glo contained about 90-95 percent less of certain toxicants than did cigarette smoke.
The company said that previous studies had shown that the biological impact of glo vapor on cells tested in the laboratory was much less than the impact of cigarette smoke on similar cells. But, it added, the reversibility of the damage following switching to a product such as glo had not been extensively studied.
“Products like glo are very new, so understanding the biological impact of vapor from glo and how that compares to cigarette smoke is a core component of our scientific research,” said Dr. James Murphy, head of reduced risk substantiation at BAT.
In the study, human airway cells were exposed repeatedly to either cigarette smoke or vapor over four weeks. For the first two weeks, the lung tissue was exposed to cigarette smoke for 15 minutes at a time, three times a week. The exposed tissue was then split into three groups: one group continued to be exposed repeatedly only to cigarette smoke for a further two weeks; a second group was exposed repeatedly to glo vapor and the third group was exposed only to air. The results obtained were then compared to results obtained by exposing airway tissue only to air for the full four weeks.
‘The results show that switching completely to glo after two weeks of repeated exposure to cigarette smoke reversed some of the biological impacts of the smoke,’ the press note said. ‘Significant reductions were observed in the amount of certain molecules produced in response to inflammation, for example, in comparison to that seen in lung tissue exposed to cigarette smoke for the full four-week period.’
BAT said the results added to evidence suggesting that glo might have the potential to be reduced risk compared to conventional cigarettes.
“We have developed a suite of tests to assess our next generation products, because we know it is by taking the results of all these tests together that gives us a real feel for the bigger picture and the potential for glo to be reduced risk compared to a conventional cigarette,” said Murphy.
The results are being presented today at the annual conference of the Society of Toxicology in San Antonio, Texas, US.
Reducing risk reduction
A smokers’ lobby group has criticised the UK government’s plan, revealed yesterday, to introduce an excise tax on heated tobacco products.
According to the government, the duty on these products will be based on the weight of tobacco in the product.
“Heated tobacco may not be as safe as electronic cigarettes but current evidence suggests there is almost certain to be a reduction in risk for cigarette smokers,” said Simon Clark (pictured), director of Forest [Freedom Organisation for the Right to Enjoy Smoking Tobacco].
“Why would any government want to undermine the future of a product that may encourage smokers to quit voluntary and without coercion?”
Clark pointed out that many smokers who tried electronic-cigarettes found they didn’t like them. The attraction of heated tobacco was that it filled the gap between combustible cigarettes and e-cigarettes, which don’t contain tobacco.
“Heated tobacco products are still in their infancy,” he said. “Adding excise duty will almost certainly deter many smokers from switching to a potentially safer device.”
Illegal activity recorded
As part of its ‘Suspect It? Report It!’ campaign to combat the illegal trade in tobacco, Imperial Brands has launched a mobile app for its UK representatives to log reports by retailers of sales of illicit tobacco products in their areas, according to a betterReailing.com story.
After only two months of operation, the new system is said to have increased the reporting of illegal activities by about tenfold. The system operates through the SARA (Scanning, Analysis, Response, and Evaluation) problem-solving model, which is a common approach used by community policing agencies to identify and solve repeat crimes and community problems.
More than 300 reports had been filed so far in 2018.
Better Retailing said that the strategy had already paid off, with seven successful raids by police and trading standards officers as a result of the evidence passed on through the SARA system.
In addition, more than 50 Facebook advertisements offering illicit tobacco had been removed.
Imperial anti-illicit trade manager James Hall was quoted as telling Retail Express that with all of the data being recorded in one central digital database, Imperial’s Insights & Intelligence team could track and analyse both national and regional patterns much more robustly.
E-cigs given a hearing
British American Tobacco’s chief scientific officer yesterday told a UK parliamentary committee that policymakers should maximize the potential of electronic cigarettes to provide an alternative to combustible cigarettes.
In his evidence to the Science and Technology Committee, which is holding an enquiry into e-cigarettes, Dr. Chris Proctor (pictured) focused on three key aspects of the vaping category.
Firstly, he said, there was a need for a broad range of products: a need to understand that it was not a “one-size fits all” situation. ‘Over the last 4-5 years we have seen strong growth in the e-cigarette category, but different consumers have different needs,’ Proctor was quoted in a BAT press note as having told the committee. ‘While e-cigarettes work for many, we need to recognise that other products, such as tobacco heating products, can also play a part. Today, at BAT we have an unrivalled range of exciting and innovative products across the potentially reduced risk categories including industry-leading products in vapor, tobacco heating products, oral tobacco, tobacco-free nicotine pouches, and moist snuff.’
Secondly, Proctor said, there was a need to address marketing restrictions because BAT believed there was a lack of public understanding about these new products that could be holding the category back. It was crucial, he added, that there was appropriate regulation in place to allow sensible marketing freedoms to give consumers the information they needed.
Thirdly, there was a need for greater focus and emphasis on product standards and quality. There was a need for quality and product-safety standards that could become the benchmark for the industry and regulators. It was only with universal standards that consumers would get the quality assurances they rightly needed.
After his committee presentation, Proctor issued a statement that welcomed the opportunity to give evidence before the committee and that underlined the need for officially-recognized product standards. “For many years, and based on our own extensive and continued research, testing and development of our products, we have highlighted that smokeless products – like vaping and tobacco heating products – can be a potentially reduced risk alternative to smoking,” he said. “This is why we have, and continue to, invest heavily in a portfolio of innovative, high-quality next-generation products, accelerating our ambition to transform tobacco.
“The UK is the largest vaping market in Europe and we believe this is largely due to its pragmatic and progressive approach to this category. However, there is still more to be done. Whilst it is great that there is increasing debate around these new products and more supportive science, we need to recognise that not all products within the category are the same. It is crucial that there is appropriate regulation in place to ensure high product standards and quality, whilst also giving sensible innovation and marketing freedoms. Only with these industry standards, will consumers get the information and quality assurances they rightly need.”
Another glowing report
A clinical study conducted by scientists at British American Tobacco has revealed that when smokers switch completely from cigarettes to the tobacco-heating product (THP) glo, their exposure to certain cigarette smoke toxicants is significantly reduced, in some cases to levels comparable to those seen in smokers who quit smoking completely.
The company points out in a footnote that the results of the study do not necessarily mean that glo is less harmful than are other tobacco products.
It says, however, that the results add to ‘evidence suggesting that [using] glo may have the potential to be substantially reduced risk compared to smoking conventional cigarettes’.
A BAT press note said that glo was designed to heat rather than burn tobacco, which meant that it did not produce smoke, and that certain toxicants associated with tobacco combustion were substantially reduced. Previous studies had revealed that toxicant levels in the vapor from glo were about 90-95 percent lower than they were in cigarette smoke.
“Products like glo are very new and consumers and regulators alike understandably want as much information as possible about them,” said Dr. James Murphy, head of reduced risk substantiation at BAT. “Understanding how vapor from glo compares to cigarette smoke is, therefore, a core component of our scientific research. Clinical studies, which are studies involving real people, are an extremely important component of that.”
The press note said that because glo vapor had lower levels of toxicants than did cigarette smoke, it should in principle expose consumers to much lower levels of toxicants.
The results of this study, which were due to be presented on Saturday at the annual conference of the Society for Nicotine and Tobacco Research in Baltimore, Maryland, US, indicated that this was the case.
BAT volumes increased
British American Tobacco’s cigarette and tobacco-heating product (THP) volumes during the 12 months to the end of December, at 686 billion, were increased by about 3.2 percent on those of the 12 months to the end of December 2016, 665 billion. On an organic basis, volumes fell by about 2.6 percent.
Tobacco volumes which include as well as cigarettes and THPs, other tobacco products whose volumes are stated in cigarette stick equivalents, were increased by about 3.6 percent, from 689 billion to 714 billion.
In a preliminary announcement about its results for the year to the end of December 2017, which saw it complete its acquisition of Reynolds American Tobacco in July, BAT said that its market share in its key markets had increased last year by 0.4 of a percentage point. This growth was said to have been driven by the group’s global drive brands (GDB), including THPs, whose market share, excluding the US, had increased by 1.1 percentage points on volume up by 7.6 percent on an organic basis.
The company’s cigarette and THP volumes were increased in its Western Europe region from 120 billion to 122 billion. But they were down in its EEMEA (Eastern Europe, Middle East and Africa) region from 236 billion to 228 billion, down in its Americas region from 113 billion to 107 billion, and down in its Asia-Pacific region from 196 billion to 193 billion.
BAT’s revenue during the year to the end of December, at £20,292 million, was increased by 37.6 percent on that of 2016, £14,751 million; while adjusted organic revenue, at £15,712 million, was increased by 6.5 percent.
Profit from operations, at £6,476, was increased by 39.1 percent; while adjusted organic profit from operations was up by 7.8 percent to £5,910 million.
Diluted earnings per share were up by 634.0 percent to 1,830.0p; while adjusted diluted earnings per share were increased by 14.9 percent to 284.4p.
Dividend per share was up by 15.2 percent to 195.2p.
“The Group delivered another set of strong financial results in 2017, despite a challenging trading environment,” said chief executive Nicandro Durante (pictured). “Following the transformational deal in July 2017, these results benefit from the acquisition of RAI while also demonstrating the strength of the organic business.”
Durante said also that BAT had made “excellent progress” with its next generation product business. “Our flagship THP, glo, first launched in Japan in December 2016, reached 3.6 percent market share by the end of 2017 – having been rolled out nationally from October 2017. Since then, 50 percent of the overall category growth in Japan has been from glo – demonstrating its strong consumer appeal in a very short period. Good initial progress is also being made in our other launch markets of South Korea, Russia, Canada, Romania and Switzerland.
“In the vapor category, Vype is now present in nine markets and we remain market leader in the UK, with Vype and Ten Motives combined delivering around 40 percent share of measured retail in December 2017. We also lead the vapor category in Poland. In the US, the Vuse range of products continues to have a significant presence in the market. We see the rapidly developing vapor category, as a whole, contributing significantly to our long-term growth ambitions in NGPs.”
New IQOS store in London
Philip Morris Limited is scheduled today to launch its fourth IQOS store in London, UK – this one in High Street Kensington.
The new store will offer adult smokers, across 800 sq ft of retail space, seven days a week, the opportunity to have guided trials by trained IQOS staff to learn about how the heated tobacco product works, and to discuss how they can switch from smoking cigarettes to IQOS.
The store will include, too, an interactive educational display to provide more information on heated tobacco technology and the IQOS product.
Customers will be able to acquire personalised and embossed IQOS devices and accessories.
The new store, which was designed by iD, uses white, grey- and copper-toned design elements to retain and accentuate the original features of the building, which was previously occupied by a large bank.
The windows of the store use a mixture of digital and feature displays, in neutral tones, to accent the stone facade of the building.
“This is a significant development in IQOS’s commercial expansion in the UK and we are delighted to be bringing the unique, retail experience that our IQOS stores deliver to High Street Kensington,” said Peter Nixon, MD of Philip Morris Limited UK & Ireland. “As a company, PMI has never previously had retail stores in the UK and so the opening of our fourth store marks an important moment for us and our ambition for a smoke free future.”
In a press note, PM said the new store marked a further development in the pledge to convert 100,000 UK adult smokers to its heated tobacco product, IQOS, and to reach its goal of a smoke free future for the UK.
‘This pledge is part of Philip Morris International’s … global commitment to offer adult smokers a range of alternative smoke free products for those who continue to smoke…’
London’s first IQOS store opened on Wardour Street, Soho, in December 2016, while the second and third stores opened in Westfield and Boxpark Shoreditch in September last year.Vaping key to quitting
Vaping poses only a small fraction of the risks of smoking and switching completely from smoking to vaping conveys substantial health benefits, according to a new Public Health England (PHE) electronic-cigarette evidence review.
The report, which was undertaken by leading independent tobacco experts, provides an update on PHE’s 2015 review.
It covers e-cigarette use among young people and adults, public attitudes, the impact on quitting smoking, an update on risks to health and the role of nicotine. It also reviews heated tobacco products.
PHE lists the report’s key findings as:
* E-cigarettes could be contributing to at least 20,000 successful new quits per year and possibly many more;
* E-cigarette use is associated with improved quit success rates over the last year and an accelerated drop in smoking rates across the country;
* Many thousands of smokers incorrectly believe that vaping is as harmful as smoking; around 40 percent of smokers have not even tried an e-cigarette;
* There is much public misunderstanding about nicotine. Less than 10 percent of adults understand that most of the harms to health from smoking are not caused by nicotine;
* The use of e-cigarettes in the UK has plateaued over the last few years at just under three million;
* The evidence does not support the concern that e-cigarettes are a route into smoking among young people. Youth smoking rates in the UK continue to decline. Regular use is rare and is almost entirely confined to those who have smoked.
PHE’s evidence review comes a few weeks after a US National Academies of Sciences, Engineering and Medicine report on e-cigarettes found that, based on the available evidence ‘e-cigarettes are likely to be far less harmful than combustible tobacco cigarettes’.
Professor John Newton, Director for Health Improvement at PHE said that smoking led to someone being admitted to hospital every minute in England, and that there were about 79,000 smoking-related deaths a year in England alone.
“Our new review reinforces the finding that vaping is a fraction of the risk of smoking, at least 95 percent less harmful, and of negligible risk to bystanders,” he said. “Yet over half of smokers either falsely believe that vaping is as harmful as smoking or just don’t know.
“It would be tragic if thousands of smokers who could quit with the help of an e-cigarette are being put off due to false fears about their safety.”
David O’Reilly, British American Tobacco’s group scientific and R&D director, welcomed the report.
“We welcome this latest report from Public Health England which reiterates their view that e-cigarettes are less harmful than smoking; that accurate information is needed about these new products; and that the evidence does not support that e-cigarettes are a gateway to smoking, and may in fact be an important tool to help people quitting,” he said. “It’s positive to see that for the first time they’ve also referenced tobacco heating products [THPs] – and how the available information suggests that these may also be considerably less harmful than traditional cigarettes.
“The report noted that there is significant public misunderstanding about risks associated with vaping and this has coincided with a plateauing of use of e-cigarettes in the U.K. We believe that this lack of understanding could be holding back this important consumer category – consumers and regulators need accurate information to provide them with the facts they need on the potential safety profile of these products. We believe the industry, public health and regulators have a role to play in providing accurate and robust information to support this important category.
“The science we’ve done on our products, across e-cigarettes and tobacco heating products, is pointing in the direction of these being a potentially safer alternative to cigarettes. We all agree that more long-term data is needed and, in line with this, at BAT, we continually assess our products, with many long-term studies currently underway across vapour and THP with our Vype and Glo brands respectively.
“Tobacco harm reduction is a critical part of our company’s strategy. We are committed to offering consumers a choice of high quality, innovative and inspiring alternative products with reduced risk potential, from vapor to THP. With increasing evidence in support of e-cigarettes, as an option for smokers looking for potentially safer alternatives, it is crucial that there is appropriate regulation in place to give consumers the information they need. It is imperative that regulations ensure high product quality and give sensible innovation and marketing freedoms, whilst also ensuring that these products are not available to youth.
“We’ve invested $2.5 billion in this important consumer category over the last six years and our commitment to the future is larger still as we seek to transform tobacco. Our quest to offer more alternatives to cigarettes, with harm reduction potential, could transform tobacco for consumers, regulators and society.”

A glowing report on vapor
Scientists at British American Tobacco have reported that they observed changes in just two genes when human airway tissue was exposed to vapor from the company’s glo tobacco heating product (THP), whereas thousands of gene changes were observed in tissue exposed to cigarette smoke.
They pointed out, however, that these results do not necessarily mean that the use of glo is less harmful than is the use of other tobacco products.
‘The impact on tissue exposed to glo vapor was minimal and more comparable with that of air when tested in laboratory conditions,’ according to a BAT press note.
‘These results add to evidence suggesting that glo has the potential to be substantially reduced risk compared to smoking conventional cigarettes.’
As part of the press note, Dr. James Murphy, head of reduced risk substantiation at BAT was quoted as saying that products such as glo were new, and that consumers and regulators wanted as much information about them as possible. That was why testing the impact of glo vapor compared to that of smoke was so important.
In this case, scientists were observing gene expression, which could give an indication of whether exposure to an aerosol, such as smoke or glo vapor, had had particular toxic effects.
BAT’s results clearly showed that cigarette smoke triggered a robust gene expression response, while exposure to vapor from glo had very limited impact on gene expression. Murphy said there was a striking difference.
In the recent study, scientists at BAT used human cells grown in the laboratory to test the impact of glo vapor and compare it to the impact of smoke and air.
The tissue (MucilAir™) is made up of human cells that grow in the laboratory to create a 3-dimensional structure that mimics the natural structure and characteristics of the living human airway. The resulting tissue is, for example, capable of producing mucus, as in the living airway, and it is covered in hair-like projections called cilia, which are used to expel inhaled dust from the respiratory system.
‘Using a robot that mimics how consumers use their products, this tissue was exposed to air, smoke from a reference cigarette (3R4F), or vapor from glo continuously for one hour,’ the BAT note said. ‘Then, to measure the cell response, the scientists mapped the genes that were switched on and off at 24 hours and 48 hours after the one-hour exposure.
‘This involves breaking open the cells and the cell nucleus to get at the genetic material inside it. The material is then studied to determine what genes are impacted.’
“Our technology is state-of-the-art,” said Murphy. “We have the capability to profile the activity of tens of thousands of genes simultaneously, providing more information than ever before on the genetic profile of exposed cells.”
‘Results show that cigarette smoke triggered thousands (2809) of changes in the expression of genes strongly involved in the development of lung cancer, inflammation and fibrosis,’ the note said. ‘In contrast, only two genes were affected by exposure to glo vapor.
‘These results, which are published in the journal Scientific Reports (doi: 10.1038/s41598-018-19627-0) add to evidence that glo vapor may cause less damage to cells as compared to cigarette smoke. Future studies will look at the impact on human tissues of more intense and longer exposure to this vapor.
‘Previous research conducted by British American Tobacco has shown that glo vapor contains around 90-95 percent less toxicants compared to cigarette smoke from a reference cigarette, in terms of the priority list of nine toxicants that the World Health Organization recommends reducing in cigarette smoke.’