During a recent product launch broadcast live worldwide on June 29, ZoVoo released four new products, its Gene Tree special edition ceramic core technology, and ZoVoo shared information on its peak performance in the electronic atomization field.
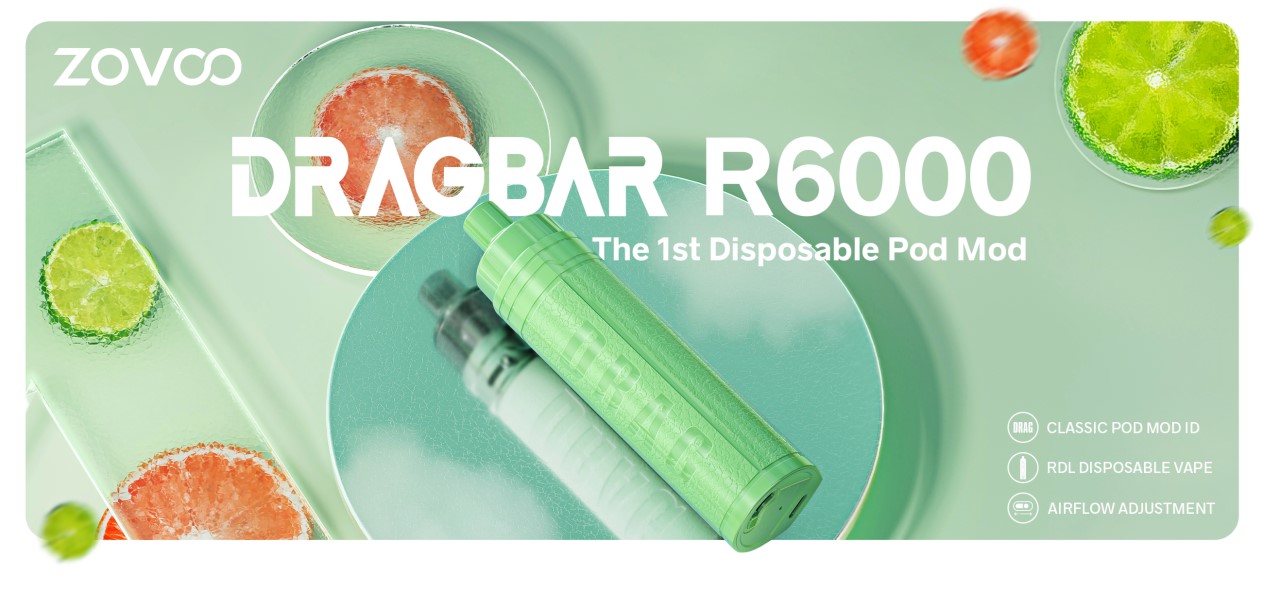
The first disposable product in the DRAG family, the new DRAGBAR R6000 is ZoVoo’s pod-style disposable. It has a classic leather texture design and its new airflow adjustment function, according to a press release.
ZoVoo also launched its lightest and thinnest ceramic core disposable product, the DRAGBAR Z700 GT, which “adopts the latest upgraded ceramic core technology, and reconstructs a new thin and light experience,” according to a release.
The new core is the latest nano-microcrystalline ceramic core independently developed by ZoVoo, GENE TREE Special Edition is the first “powder free” ceramic core with proprietary patented technology in the industry, according to the release.
Also launched, DRAGBAR F8000, with max 8000 puffs, adopts the infinite airflow adjustment system, and matches with high-performance of a mesh coil. Also, the new VINCIBAR F2500 adopts Kevlar texture, integrates light and shadow aesthetics, elegant and comfortable. With max 2500 puffs, 15 flavors and mesh coil design.







 The U.S. Food and Drug Administration on May 12
The U.S. Food and Drug Administration on May 12 